Oxidation numbers of according to the atomic number, Here we are providing some elements. Oxidation state can be defined as the number of electrons that a particular atom can lose, gain or share with another atom.
Chemistry: Table of Oxidation Numbers of Elements
Today we will learn about chemistry and topic is Table of Oxidation Numbers of Elements.
About oxidation state
Oxidation state can be defined as the number of electrons that a particular atom can lose, gain or share with another atom. The loss or gain of electrons changes the charge of the atom because electrons have a negative charge, and each negative charge is neutralized by the positive charge of the protons in the nucleus.
An atom is losing or gaining electrons
When an atom is losing or gaining electrons, there will be an imbalance of electric charges. Therefore, the oxidation state of that atom is the charge of that atom. Oxidation state can be used to describe the charge of an atom present in a compound.
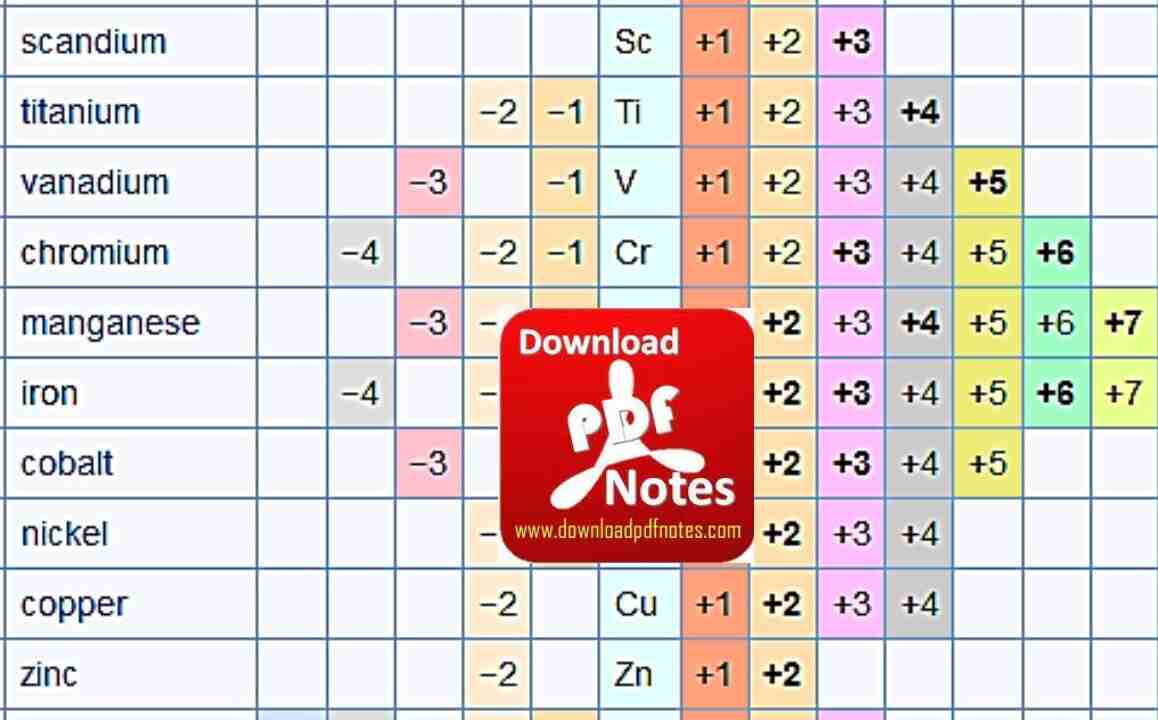
Table of Some Oxidation Numbers of Elements
Table-1
|
Element/Ion/Radical |
Symbol |
Valence or Oxidation Number |
|
Aluminum |
Al |
3+ |
|
Antimony |
Sb |
3+, 5+ |
|
Arsenic |
As |
3+, 5+ |
|
Barium |
Ba |
2+ |
|
Bismuth |
Bi |
3+ |
|
Boron |
B |
3+ |
|
Bromine |
Br |
1- |
|
Calcium |
Ca |
2+ |
|
Cadmium |
Cd |
2+ |
|
Carbon |
C |
2+, 4+ |
|
Chlorine |
Cl |
1- |
|
Chromium |
Cr |
2+, 3+, 6+ |
|
Cobalt |
Co |
2+, 3+ |
|
Copper |
Cu |
1+, 2+ |
|
Fluorine |
F |
1- |
|
Hydrogen |
H |
1+ |
|
Iodine |
I |
1- |
|
Iron |
Fe |
2+,3+ |
|
Lithium |
Li |
1+ |
|
Lead |
Pb |
2+, 4+ |
|
Magnesium |
Mg |
2+ |
|
Manganese |
Mn |
2+, 4+, 7+ |
|
Mercury |
Hg |
1+, 2+ |
|
Nickel |
Ni |
2+ |
|
Nitrogen |
N |
3- ,3+, 5+ |
|
Oxygen |
O |
2- |
|
Phosphorous |
P |
3+, 5+ |
Table-2
|
Element/Ion/Radical |
Symbol |
Valence/Oxidation Number |
|
Potassium |
K |
1+ |
|
Silicon |
Si |
4+ |
|
Silver |
Ag |
1+ |
|
Sodium |
Na |
1+ |
|
Strontium |
Sr |
2+ |
|
Sulfur |
S |
2-, 4+, 6+ |
|
Tin |
Sn |
2+, 4+ |
|
Zinc |
Zn |
2+ |
|
Gold |
Au |
1+, 3+ |
|
Acetate |
C2H3O2 |
1- |
|
Bromate |
BrO3 |
1- |
|
Bromic Acid |
HBrO3 |
1- |
|
Bromous Acid |
HBrO2 |
1- |
|
Bromite |
BrO |
1- |
|
Carbonate |
CO3 |
2- |
|
Chlorate |
ClO3 |
1- |
|
Chlorite |
ClO2 |
1- |
|
Chromate |
CrO4 |
2- |
|
Cyanide |
CN |
1- |
|
Dichromate |
Cr2O7 |
2- |
|
Hydride |
H |
1- |
|
Hydrogen Carbonate/ Bicarbonate |
HCO3 |
1- |
|
Hydrogen Sulfate/ Bisulfate |
HSO4 |
1- |
|
Hydrogen Sulfite/ Bisulfite |
HSO3 |
1- |
Table-3
|
Element/Ion/Radical |
Symbol |
Valence/Oxidation Number |
|
Hydroxide |
OH |
1- |
|
Hypochlorite |
ClO |
1- |
|
Nitrate |
NO3 |
1- |
|
Nitrite |
NO2 |
1- |
|
Perchlorate |
ClO4 |
1- |
|
Permanganate |
MnO4 |
1- |
|
Peroxide |
O2 |
2- |
|
Phosphate |
PO4 |
3- |
|
Phosphite |
PO3 |
3- |
|
Sulfate |
SO4 |
2- |
|
Sulfite |
SO3 |
2- |
|
Thiocyanate |
CNS |
1- |
|
Iodate |
IO3 |
1- |
|
Thiosulfate |
S2O3 |
2- |
|
Oxalate |
C2O4 |
2- |
|
Silicate |
SiO3 |
2- |
|
Arsenate |
AsO4 |
3- |
|
Borate |
BO3 |
3- |
|
Ferricyanide |
Fe(CN)6 |
3- |
|
Ammonium |
NH4 |
1+ |
|
Hydronium |
H3O |
1+ |

![[PDF*] Navy MR Complete Study Material Books | Practice Set | Previous Year Paper | Mock Test Free](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEju1OtWAETNykdIMMl1EccY2golN8cIsZ6kuKw89_a63nGAYBg2hGx_cn33HhLBgy8dKG_PM5Kamkr0Iazf2gkEDbCMhornMbFLiHxo33Qu7ToC3cfpjr6X_RFAZym3KmdUhVOKmFtZQ_w/w72-h72-p-k-no-nu/1010.jpg)
0 Comments
Thanks for comment!